Coastal Acidification Varies Seasonally, Daily, and Among Locations in Casco Bay
Scientists Are Investigating Local Patterns and Causes of Acidification
WHY IT MATTERS
Lobsters, clams, and many other marine species build their shells out of carbonate minerals that they extract from seawater. Acidification of the water can make it more difficult for them to build their shells, or even cause shells to dissolve. Four out of every five dollars in Maine fisheries comes from shell-building species. Lobster represents about 75 percent of the fisheries’ total value, and clams, oysters, urchin, and scallops account for another five or six percent.
In offshore waters, the primary cause of acidification is carbon dioxide (CO₂) from the atmosphere dissolving into the water. One quarter of the carbon dioxide released to the atmosphere by human activity annually is absorbed by the ocean, making ocean acidification a global concern. Near the coast, however, other localized sources such as rivers and nutrient pollution are major contributors to acidification, which refers to changes in not only pH but also availability of carbonate ions. Coastal acidification interacts with other environmental stressors like climate change, invasive species, and poor water quality to affect the ecosystem.
STATUS & TRENDS
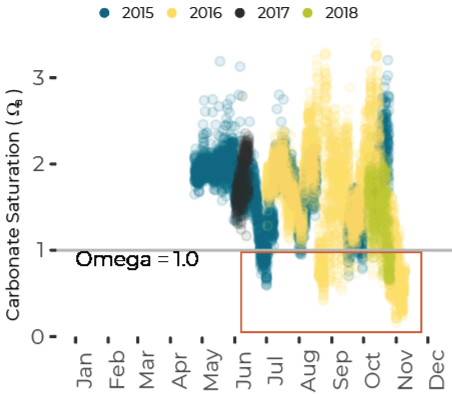
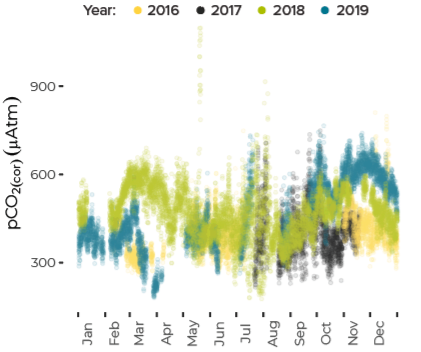
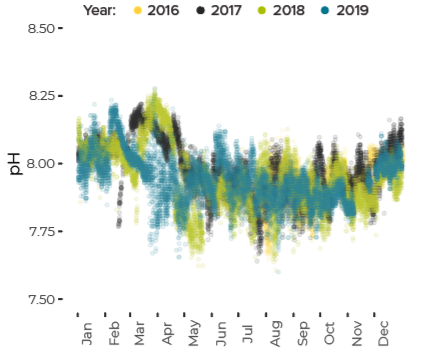
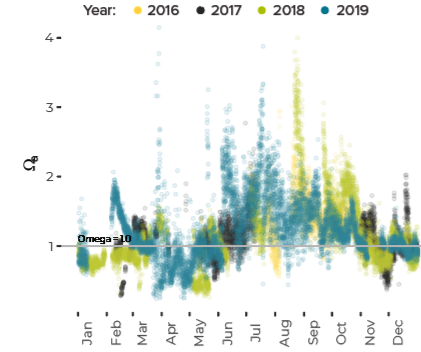
Data are limited, especially for the winter months, but about ten percent of all observations in Casco Bay show conditions corrosive enough to dissolve carbonate minerals like clam shells. Scientists from the University of New Hampshire, with funding through Casco Bay Estuary Partnership, have monitored coastal acidification near Southern Maine Community College (SMCC) in South Portland for five years. The monitoring reveals seasonal and daily variations reflecting biological activity in the ecosystem. Carbon dioxide levels fluctuate seasonally and daily, in synchrony with cycles of photosynthesis, respiration, and decomposition. In late summer and fall, levels of carbon dioxide hit their peak, pH drops, and acidification reaches levels of concern. For most of the year, levels of carbon dioxide dissolved in the Bay’s waters are higher than atmospheric levels, meaning the Bay seldom absorbs carbon dioxide from the atmosphere.Dissolved Carbon Dioxide Rises over the Summer and Fall
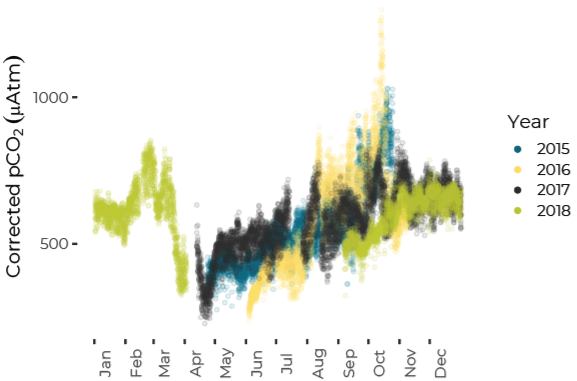
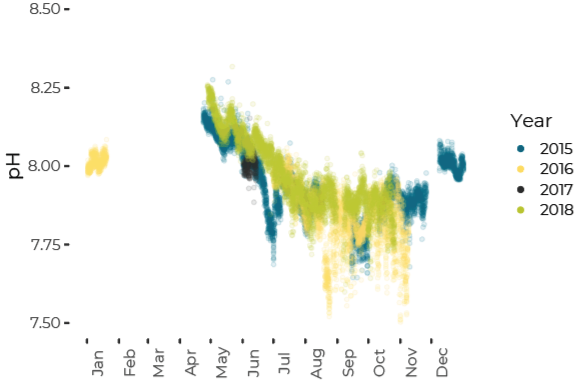
pH Decreases from Summer to Fall
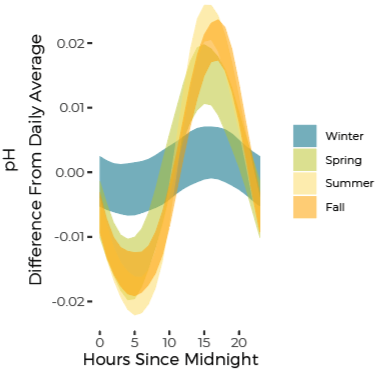
pH Fluctuates Through Day and Night Reflecting Biological Activity
Potential for Shell Corrosion in Late Summer and Fall
Monitoring by Friends of Casco Bay
Since 2016, Friends of Casco Bay (FOCB) has monitored acidification parameters from a location on Cousins Island in Yarmouth. Results are similar, but not identical, to findings from SMCC.
successes & challenges
- The Gulf of Maine, including Casco Bay, is especially vulnerable to acidification because of its cooler waters and the low alkalinity of the region’s freshwater inputs.
- Coastal acidification is already affecting shellfish aquaculture in Maine, especially in the Damariscotta River, where freshwater inflows make the system vulnerable.
- Monitoring of acidification in Casco Bay and other estuaries is revealing how local conditions affect acidification. In Casco Bay, data suggest that acidification may be affected by tides and by river discharge, but the science remains inconclusive for now.
- Laboratory experiments have demonstrated that levels of acidification that are observed from time to time in Casco Bay can affect behavior and reproduction of softshell clams and quahogs. However, it is not yet known whether present-day conditions affect shellfish living in the wild.
- Monitoring in other estuaries with more nutrient pollution than Casco Bay has revealed high levels of dissolved carbon dioxide and more dramatic daily and seasonal fluctuations. Reducing nutrient pollution could help protect coastal ecosystems from acidification.
View a PDF version of this page that can be downloaded and printed.
View references, further reading, and a summary of methods and data sources.
STATE OF CASCO BAY
Drivers & Stressors
What’s Affecting the Bay?
Human Connections
What’s Being Done?
If you would like to receive a printed State of Casco Bay report, send an email request to cbep@maine.edu.
This document has been funded by the U.S. Environmental Protection Agency under Cooperative Agreements #CE00A00348-0 and #CE00A00662-0 with the University of Southern Maine.
Suggested citation: Casco Bay Estuary Partnership. State of Casco Bay, 6th Edition (2021).
Photo at top of page: Jerry Monkman, Ecophotography.com